HPV Testing
HPV FAQ’s
When does HPV Primary Screening go live?
The current date set by the NCSP to change to HPV testing is 12th September 2023.
Up until this date please ensure you continue to collect a cervical cytology specimen in the LBC vial.
Which laboratories will be providing the HPV Primary Screening test?
The NCSP selected three laboratory service providers to perform HPV Primary screening across New Zealand - Pathlab, Awanui Labs (formerly SCL), and LabPlus (APS). Pathlab will be providing HPV testing for the following regions:
- Waikato
- Bay of Plenty
- Lakes
- Tairawhiti
- Hawkes Bay
- MidCentral
- Whanganui
Will I need to change the liquid-based cytology (LBC) vial I use?
The NCSP has chosen SurePath as the LBC. This means if you are currently using ThinPrep you need to transition to SurePath.
When is the change to SurePath LBC occurring?
For those regions currently using ThinPrep LBC vials (Tairawhiti, Hawkes Bay, MidCentral, Whanganui) you must have changed over to SurePath LBC vials from 1st July 2023.
What do I do with the ThinPrep vials I have remaining at the time of change over to SurePath?
The unused ThinPrep vials should be packaged up in a separate container to patient specimens and returned to Medlab Central.
How do I order HPV Primary Screening specimen consumables from Pathlab?
Please follow directions for ordering supplies as per this 'Order Supplies' page under the Providers tab on our website - https://www.pathlab.co.nz/supplies.
How do I send the collected HPV screening specimens to Pathlab?
Transportation of specimens is as per your current courier service.
What are the minimum labelling requirements for an HPV specimen?
HPV specimens are like all laboratory specimens and require a minimum of at least 3 identifying criteria which can include: first name, last name, NHI number, date of birth. If there are fewer than 3 unique identifiers on the specimen, a recollect may be required.
Do I need a separate laboratory form for the HPV specimen and other specimens that may be collected at the same time?
If you use electronic ordering then the existing on-line form will be updated to reflect the new HPV screening specimens and tests from 12th September.
If you use paper request forms then there will be two new printable forms that can be downloaded from the NCSP website, one that can be used for LBC’s or HPV swabs to order either or both tests, and a simpler version if HPV testing is being ordered on just a swab, (less information required).
What specimen types can I use for HPV screening?
The introduction of HPV as the primary test enables the use of a vaginal swab either self-collected by the patient or clinician collected, or a SurePath liquid based cytology (LBC) cervical collection. It is anticipated a large number of patients will elect to have a swab collect rather than an LBC.
It should be noted that in some clinical circumstances an LBC would be the recommended specimen, e.g. if the patient is symptomatic, for some follow-up testing, if co-testing is required.
Can the patient elect to have a cytology screen rather than an HPV test?
No. The new screening test is HPV which is far more sensitive than cytology as a screening test for cervical cancer.
However, if the HPV test is positive and an LBC specimen was collected then it will automatically get cytology reflex testing. If a swab is HPV positive, then an LBC collect will be requested so cytology testing can be performed.
What specimen should be collected on symptomatic patients?
If the patient is symptomatic then it is recommended an LBC is collected with the symptoms recorded on the laboratory form, and co-testing for HPV and cytology requested.
How much do the HPV collection kits cost?
HPV swab kit and SurePath vial costs are covered within the test funding and are at no cost to you as per other laboratory specimen consumables. Please ensure you only order what you need and have good stock rotation to ensure collection consumables don’t expire. Please do not use expired kits as these specimens will require a recollect.
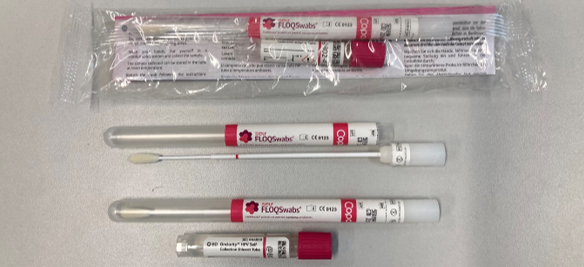
What does the HPV swab collection kit look like?
The HPV swab collection kit provided by Pathlab contains:
- Collection instructions - HERE on our website.
- Flocked swab for sampling
- Buffer tube to break off swab into.
HPV swab collection kits will be available for distribution from Monday 28th August, please order through your normal stores process. These are not to be used for HPV specimen collection until Tuesday 12th September 2023.
How quickly do I have to get the HPV specimen to the laboratory?
Once the HPV swab is in the buffer tube it is stable for 15 days at room temperature. The SurePath LBC vial is stable for 4 weeks at room temperature.
It is recommended all specimens are transported to the laboratory as soon as possible and please ensure samples are not exposed to extreme heat, e.g., don’t leave in direct sunlight or in an unoccupied car.
How do I contact Pathlab if I have any questions?
You can either phone Pathlab on 07 578 7073 or use this secure contact form on our website.
How do I label the HPV swab buffer tube?
It is important you know the swab buffer tube is placed in a rack that is then loaded into a fully automated instrument so please ensure the 2D barcodes at the top of the buffer tube label are not covered as the instrument needs to read these.
Also, if using sticky labels please ensure there are no flags or tags otherwise the tube will not fit into the instrument racks. Thank you for your assistance.
Where can I find additional information about HPV primary screening?
There is information on the National Screening Unit website, https://www.nsu.govt.nz/health-professionals/national-cervical-screening-programme, and Time to Screen website, https://www.timetoscreen.nz/cervical-screening/changes-to-the-test/.